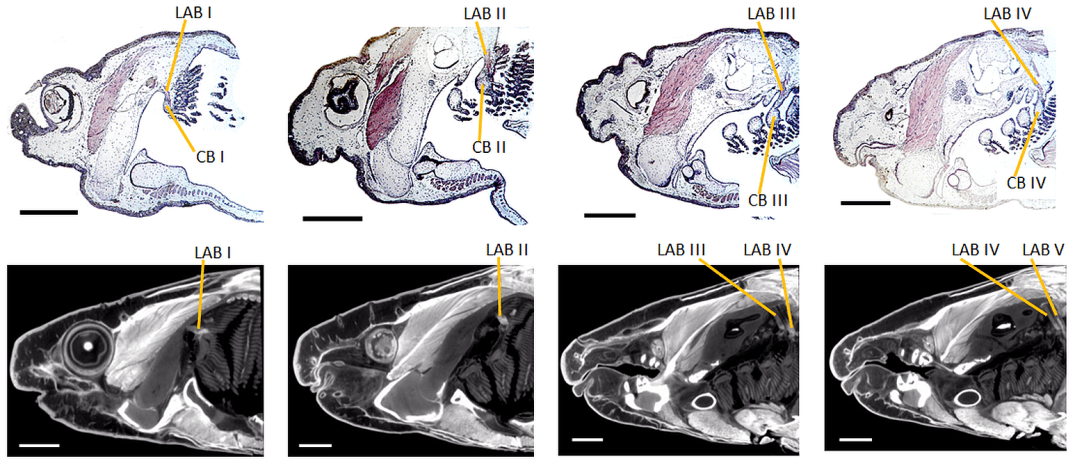
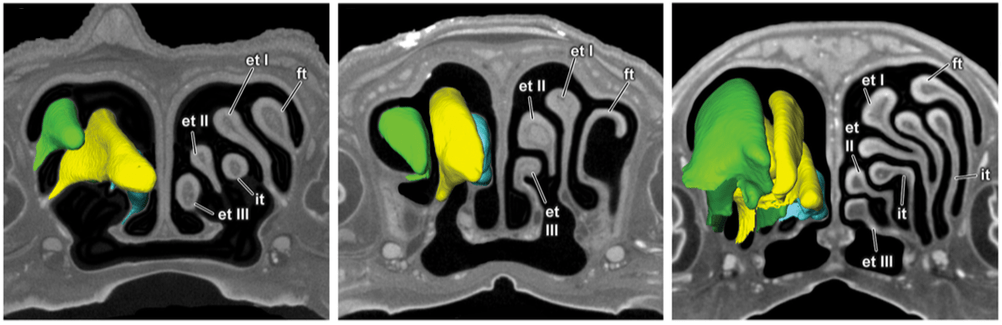
Welcome to the online home of Diffusible Iodine-based Contrast-enhanced Computed Tomography.
Our mission is to provide digital resources for the diceCT community and to connect interested researchers with contrast-enhanced imaging veterans. Watch this space and @diceCT for updates on new publications, tips & tricks, and diceCT-related events.
New Content
The Austin Working Group | 2015 SVP Workshop | 2016 ICVM Symposium | Pubs